NOW RECRUITING
The human clinical trial for olfactory nerve bridge cell transplantation combined with intensive exercise therapy has commenced on the Gold Coast, Queensland.
We are now recruiting through an Expression of Interest (EOI) process where eligible participants will be asked a series of questions to determine their suitability for the trial. The EOI can be accessed through the ‘Complete and EOI’ button below.
If you are interested in participating but not sure as to your eligibility, please read the information further down the page for details on the clinical trial inclusion and exclusion criteria, and details on frequently asked questions.
Behind the project
See into the lab and learn about the nerve bridge design
Motivations and solution
See the new treatment being trialed for individuals with spinal cord injury
This is a randomised, blinded and controlled Phase I trial to measure the safety, feasibility and efficacy of a combined cell transplantation and intensive rehabilitation intervention to treat spinal cord injury. The trial aims to examine whether transplantation of olfactory cell nerve bridges combined with intensive rehabilitation is safe and feasible for people living with chronic spinal cord injury in Australia. Assessments will also measure whether the intervention improves structural integrity of the spinal cord and functional recovery, as well as the overall health and social wellbeing of participants.
The trial will include two groups: a control group receiving only the intensive, long-term rehabilitation program, and an active group receiving both the intensive, long-term rehabilitation program and the olfactory cell nerve bridge transplantation.
Active Group Participation
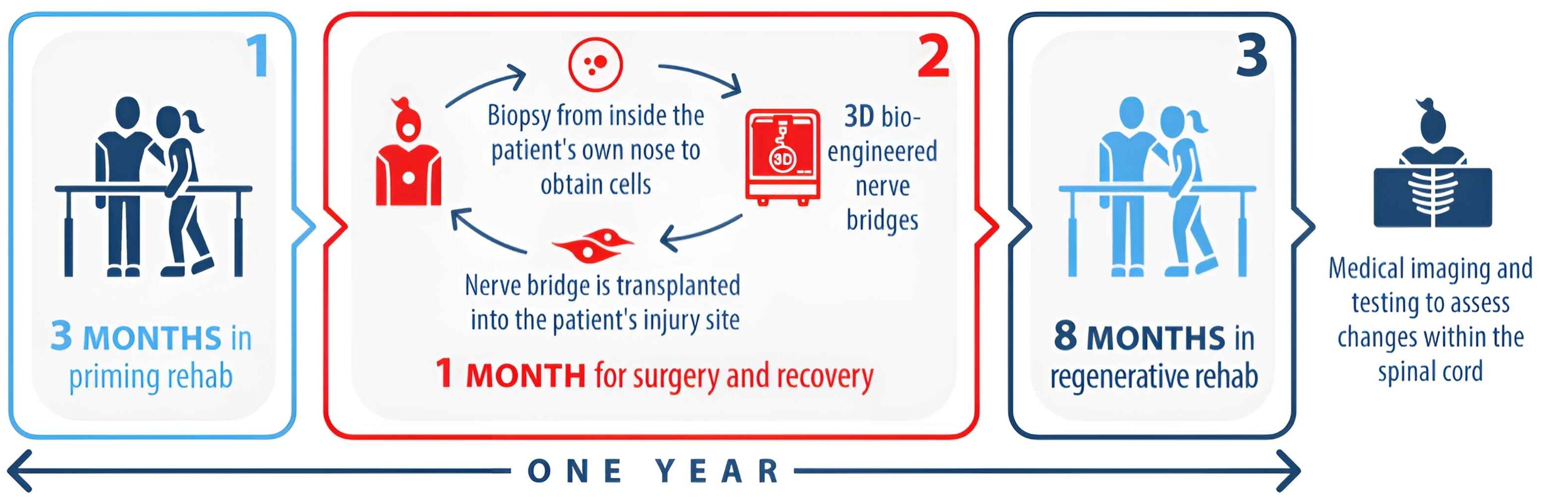
Control Group Participation

Key inclusion criteria
Participants are eligible to be included in the study only if all the following criteria apply:
- Have sustained a traumatic spinal cord injury a minimum of 4 months prior to consent and have completed their primary rehabilitation;
- Have a stable neurological level and functional ability of more than two months in duration;
- Are over 18 years and able to give informed consent;
- Are AIS A, B or low functioning C (more than 75% of key muscles have a power grade <3) as per the International Standards for Neurological Classification of spinal cord injury;
- Have a thoracic injury (T1-T12) or lower cervical (C5-C8) injury;
- Are able and willing to attend an exercise program five times per week for the duration of the priming rehabilitation program, with allowance for two weeks recreational leave;
- Are able and willing to attend an exercise program five times per week for at least 12 weeks of the regenerative rehabilitation program and then at least three days per week for the remaining 20 weeks, with allowance for four weeks recreational leave;
- Are considered by their general practitioner or specialist medical consultant to be fit to receive the cell transplantation and undertake the exercise program (documented approval by general practitioner required).
For full details and inclusion and exclusion criteria, please visit the Australia and New Zealand Clinical Trials Registration site for the trial:
About the trial
BEFORE GOING LIVE THIS MUST BE CHANGED TO 'COMPONENT | INTRODUCTION' FORMAT
This is the first in-human clinical trial of a new treatment for spinal cord injury. The study is testing effectiveness, feasibility and safety of treating spinal cord injury by olfactory cell transplantation combined with personalised intensive long-term rehabilitation. The study also seeks to determine if the treatment program improves overall health, social outcomes and functional recovery for participants. The purpose of this study is to assess if the new treatment:
- is safe and feasible,
- leads to changes in motor and sensory function, and
- improves overall health and social outcomes for participants.
The study is sponsored by Griffith University and is funded by Griffith University, the Clem Jones Foundation, the Queensland Government and numerous members of the spinal cord injury community and general public through the Perry Cross Spinal Research Foundation.
Behind the project
See into the lab and learn about the nerve bridge design
Motivations and solution
See the new treatment being trialed for individuals with spinal cord injury
About the team
Alongside researchers who work on the science behind the Spinal Injury Project, our team also includes an advisory board of medical specialists and industry experts as well as a national consumer panel of members of the spinal cord injury community. These structures have helped to design a treatment which considers both the social and medical aspects of effective care.
The advisory board of the Spinal Injury Project is Chaired by the Ear, Nose and Throat clinician Dr Chris Perry. Other members of the board include Dr Michael Redmond (neurosurgeon), Ms Frances Porter (Executive Manager, Business Development at Spinal Life Australia), Professor Lisa Harvey (University of Sydney), Mr Peter Johnstone (Clem Jones Foundation), Mr Ujjwal Dua (Griffith Enterprise, Griffith University's commercialisation office), and Dr Brent McMonagle (Perry Cross Spinal Research Foundation).
The national consumer panel includes members of the spinal cord injury community; Ashlee Morton, Brad Connelly, Dominic Beirne, Kirsty Jennings, Nick Dempsey, Steve Ralph, and Tushar Yadav who provide practical guidance and invaluable insights from their lived experience.
About the treatment
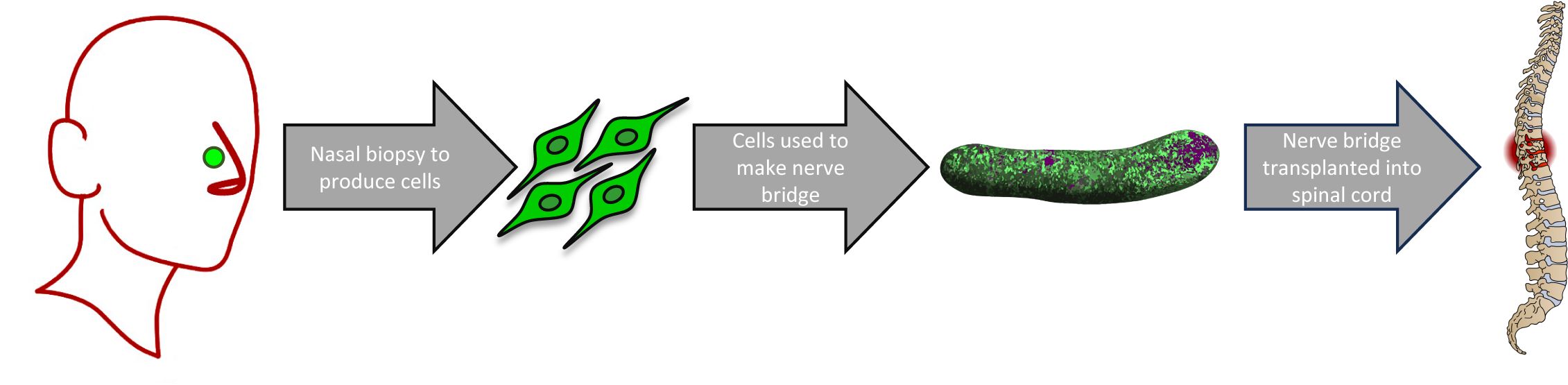
Cells are the basic structural units that make up our bodies. When an injury cannot naturally be repaired, transplanting cells that have special properties from one part of the body into the injury site can help the repair process.
In this trial, cells from the nerve responsible for sense of smell will be used for transplantation. These cells have been chosen because they have several properties that are useful for repairing a damaged spinal cord. To obtain the cells, a small biopsy will be taken from inside the nose of each participant. The biopsy will be processed to isolate the required cells, which will then be formed into three-dimensional structures that are called nerve bridges. These nerve bridges will contain only the participants own cells. When transplanted into the spinal cord injury site, cells of the nerve bridges form an environment that encourages repair and regeneration of the surrounding area.
Who is eligible to take part?
You may be able to take part in this trial if you:
- Are above 18 years of age,
- Have an acquired spinal cord injury,
- Are capable of giving signed informed consent, and
- Are able to regularly attend one of three rehabilitation providers for the trial; Making Strides in Burleigh Heads or Ormeau QLD, Royal Rehab in Ryde NSW or The Next Step Spinal Cord Injury Recovery Incorporated in Epping VIC.
Additional criteria also apply. If you wish to participate you will need to undergo a series of pre-screening tests to assess if you are eligible. This screening process will be discussed further with you if you complete an expression of interest.
What happens during the study?
This trial includes both a treatment group and a control group. For both groups;
- The trial duration will be 13 to 15 months after screening is completed.
- Trial site visits for screening, enrolment, intermittent testing and final screenings will occur at the Gold Coast University Hospital on the Gold Coast.
- Part of the screening will assess if you have metalwork in your spine and if it interferes with the medical imaging. If the clinicians think it would be safe to remove this metalwork to improve medical imaging then you will undergo surgery to remove it. If the clinicians think it is unsafe to remove the metalwork, but that it will significantly interfere with medical imaging, you may be ineligible to participate in the study.
- If you are accepted into the trial, participants will be required to attend two rehabilitation programs which will be run at their local provider (of those listed above) for 12 weeks and then for 32 weeks.
The difference between the treatment group and control group is that the control group will not undergo a nasal biopsy and will not receive the cell transplantation surgery;
If you are assigned to the treatment group, you will first undergo screening. Part of this screening will involve the metalwork identification, assessment and removal as mentioned above. If you undergo surgery to remove metalwork you will then be able to recover for 4 weeks at home, or at accommodation provided by the trial if you are from Sydney or Melbourne. After this you will commence the first rehabilitation program. This program will go for 12 weeks, and is focused on training you in the exercises and getting your body ready for the transplantation surgery.
You will then have the surgery to transplant the nerve bridges into your spinal injury site. You will have 4 weeks to recover from this at home, and then you will commence the second rehabilitation program which will go for 32 weeks. At the end of this second rehabilitation program you will have completed all active components of the trial except for a follow up visit at the Gold Coast University Hospital 2 weeks after the final session of rehabilitation.
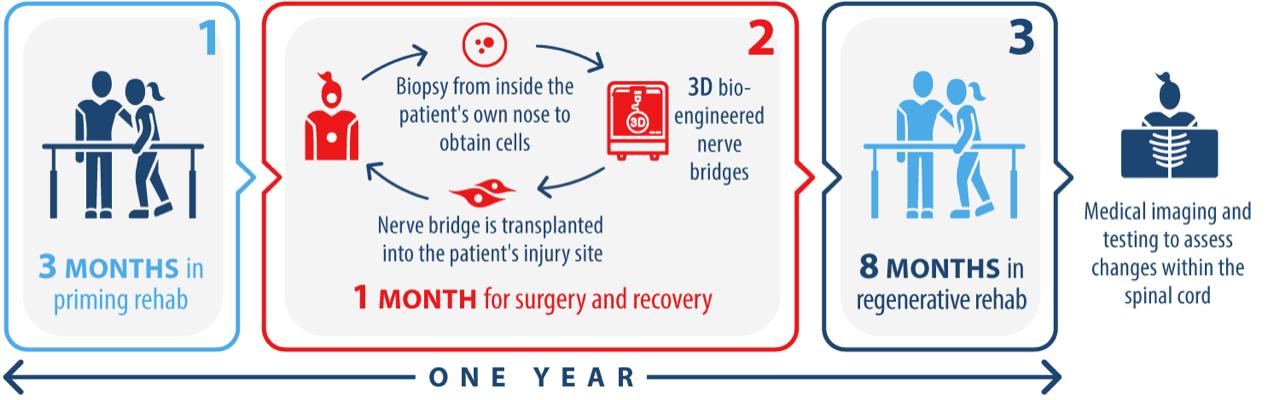
If assigned to the control group you will undergo the same screening process and removal of metalwork if safe and required as described for the treatment group. After recovery you will commence the same 12 week rehabilitation program focused on getting your body used to the exercises and fit for surgery, but you will not receive the transplantation surgery.
You will then have a break of 4 weeks with no scheduled activities. After this break you will commence the second rehabilitation program which will go for 32 weeks. At the end of the second rehabilitation program you will have completed all active components of the trial except for a follow up visit 2 weeks after the final session of rehabilitation.

What will the rehabilitation programs involve?
The first 12 week program will consist of up to 3 hours of activity-based therapy sessions per day for 5 days per week (Monday to Friday) at your local rehabilitation facility. The programs will be personalised based on the nature of your spinal cord injury and will include activities that are available in your local rehabilitation centre, such as manual manipulation methods, assistive and automated devices and functional electrical stimulation.
Due to the length and intensity of the commitment, you will be allowed to have up to two weeks of recreational leave during this period of pre-surgery rehabilitation.
The 32 week rehabilitation program is included in this study as physical activity is needed to encourage nerve regeneration and to enable repairing nerve cells to grow in the correct locations. The more activity you do, the more likely it is that connections will be established. For this reason, long-term intensive rehabilitation is required to fully assess the impact of transplanted nerve bridges.
This 32 week program will also be personalised. It will include up to 3 hours of activity-based therapy sessions per day, with no training on public holidays or when your local rehabilitation provider is closed . For the first 12 weeks of this program, you will need to attend 5 days per week (Monday to Friday). After this first 12 weeks, you can choose to continue 5-days per week, or you can reduce the program to 3-4 days per week for the remaining 20 weeks.
You will undergo physical and medical functional and psychological testing throughout both programs to monitor for improvements or adverse events linked to the trial.
You will also be given the choice to be photographed or filmed at seven timepoints over the course of the trial. These images will be used to identify your progress and to assist interpretation of the physical and medical assessments. If deemed appropriate, and if you provide prior written consent at a later date, images or video may also be shown in the media for promotional purposes (for example in newsletters, websites, television and social media).
What does the transplant surgery involve?
The surgery to transplant the nerve bridges into your spinal cord injury will be performed at Gold Coast University Hospital. It will begin with removal of some of the bone over the injury site to expose your spinal cord. The nerve bridges will then be placed into the injury site. As mentioned above, these nerve bridges contain only your own cells which were obtained from the nasal biopsy.
Additional information
Griffith University has reviewed all the study documentation and assessed the risks of this study. Griffith University is happy to take responsibility as Sponsor of this study. This study has been designed to make sure the researchers interpret the results in a fair and appropriate way and avoid study doctors or participants jumping to conclusions.
There are also no additional costs associated with participating in this study. All medication, tests, medical care and rehabilitation required as part of the study will be provided to you free of charge.
Participation in this study is completely voluntary. If you agree to participate in the trial but then change your mind, you are free to withdraw your consent at any time without it affecting your future health care.
Eligible participants will be reimbursed for their time and all associated travel will be paid for and arranged by the trial. For further information please contact Yasmin Arena-Foster at scitrial@griffith.edu.au.
The ethics approval number for the Spinal cord injury rehabilitation and nerve bridge transplant trial is HREC/2024/QGC/105231